2024-11-08
CAR-T PSCA Therapy: A New Hope for Castration-Resistant Prostate Cancer
Oncology
Castration-resistant prostate cancer (mCRPC) presents a major challenge
for healthcare professionals. Despite recent advances, treatment options for
this deadly disease remain limited. CAR-T therapy, which involves modifying
immune cells to specifically target and destroy cancer cells, has shown
promise; however, its effectiveness against solid tumors has yet to be
confirmed.
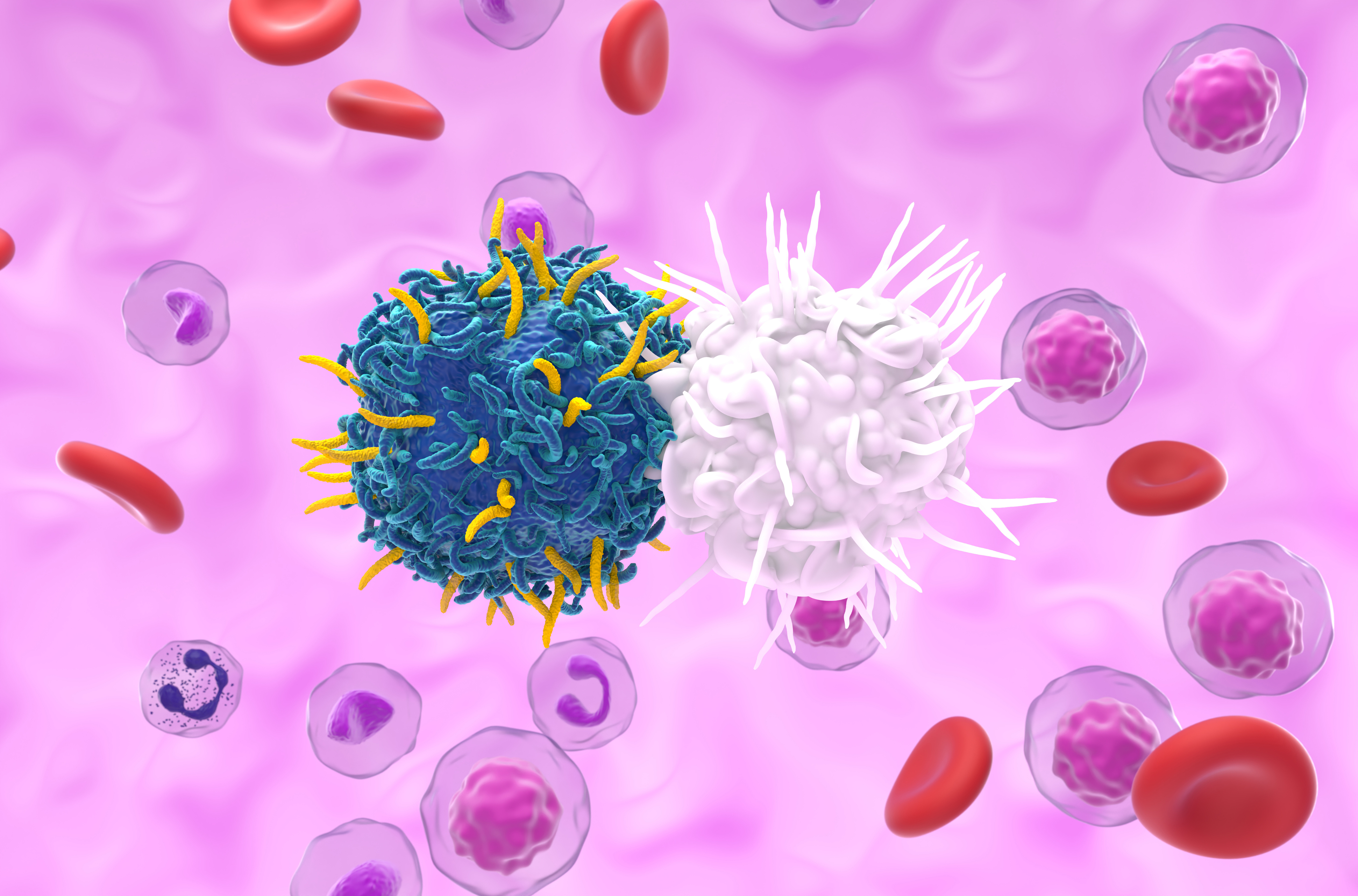
This study explores the potential of CAR-T cells in treating mCRPC,
specifically targeting the Prostate Stem Cell Antigen (PSCA). The primary
objective is to assess the efficacy, safety, and potential effects of this
approach in addressing the unmet therapeutic needs of patients with mCRPC.
In this study, 14 patients with significant PSCA expression and advanced mCRPC were divided into three cohorts, each receiving increasing doses of PSCA CAR-T cells, with some patients undergoing prior lymphodepletion (LD):
The results showed an antitumor response in 4 out of 14 patients, with a 30% reduction in prostate-specific antigen levels. Dynamic changes in the composition of peripheral blood T cells confirmed immune system activation. Radiographic improvements in some participants indicated a reduction in tumor mass. Additionally, a moderate cytokine release syndrome was observed, without severe neurological or immune toxicity. Overall, CAR-T therapy was well tolerated, with grade 3 cystitis being the most common DLT.
These findings demonstrate that CAR-T cells targeting the PSCA antigen represent a promising therapeutic approach for mCRPC. Besides inducing an effective antitumor response, the safety profile is acceptable. These results lay the groundwork for optimizing dosages and combination strategies to enhance CAR-T cell persistence. Future directions for this therapy could include adjustments in lymphodepletion, development of next-generation CAR-T cells, and exploration of additional antigen targets to maximize benefits for patients resistant to current treatments.
CAR-T PSCA Therapy: Results And Initial Clinical Observations
In this study, 14 patients with significant PSCA expression and advanced mCRPC were divided into three cohorts, each receiving increasing doses of PSCA CAR-T cells, with some patients undergoing prior lymphodepletion (LD):
- Cohort DL1: 100 million CAR-T cells without lymphodepletion.
- Cohort DL2: 100 million CAR-T cells with standard lymphodepletion.
- Cohort DL3: 100 million CAR-T cells with reduced lymphodepletion.
The results showed an antitumor response in 4 out of 14 patients, with a 30% reduction in prostate-specific antigen levels. Dynamic changes in the composition of peripheral blood T cells confirmed immune system activation. Radiographic improvements in some participants indicated a reduction in tumor mass. Additionally, a moderate cytokine release syndrome was observed, without severe neurological or immune toxicity. Overall, CAR-T therapy was well tolerated, with grade 3 cystitis being the most common DLT.
Toward Optimizing CAR-T Therapies in mCRPC
These findings demonstrate that CAR-T cells targeting the PSCA antigen represent a promising therapeutic approach for mCRPC. Besides inducing an effective antitumor response, the safety profile is acceptable. These results lay the groundwork for optimizing dosages and combination strategies to enhance CAR-T cell persistence. Future directions for this therapy could include adjustments in lymphodepletion, development of next-generation CAR-T cells, and exploration of additional antigen targets to maximize benefits for patients resistant to current treatments.
Last press reviews
Could statins soothe inflammation?

By Ana Espino | Published on February 6, 2026 | 3 min read<br>
IAVI G004 trial: overview of a next generation HIV vaccine
By Carolina Lima | Published on February 5, 2026 | 3 min read
Liver, sugar, and pills: who's in control?
By Ana Espino | Published on February 4, 2026 | 3 min read<br>