2024-09-24
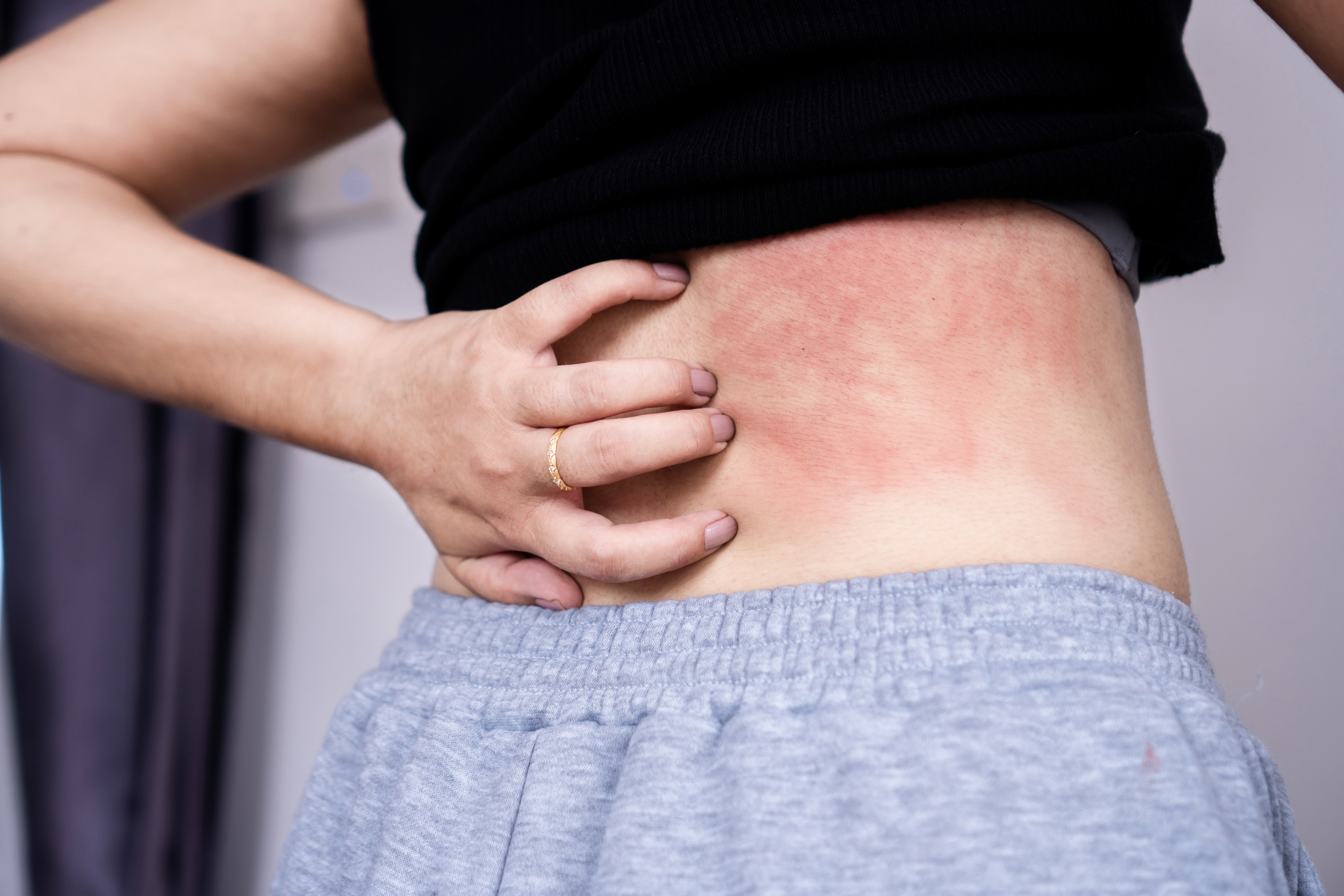
Efficacy and safety of upadacitinib in the treatment of moderate to severe atopic dermatitis in adolescents: a systematic review and meta-analysis of randomised controlled trials
Dermatology and Venereology
Source(s) :
Huang L ; Zhao D ; Lin H ; Zheng H ; Li X ; Chen L ; Tang P ;
Last press reviews
Gut microbiome and neurodegeneration: a new therapeutic lever

By Elodie Vaz | Published on February 12, 2026 | 3 min read<br>
Fragile heart, vulnerable brain?

By Ana Espino | Published on February 13, 2026 | 3 min read<br><br>
A practical look at HCM in young athletes

By Carolina Lima | Published on February 13,&nbs...