2024-03-11
Epigenetics and angiogenesis in the tumor microenvironment
Oncology
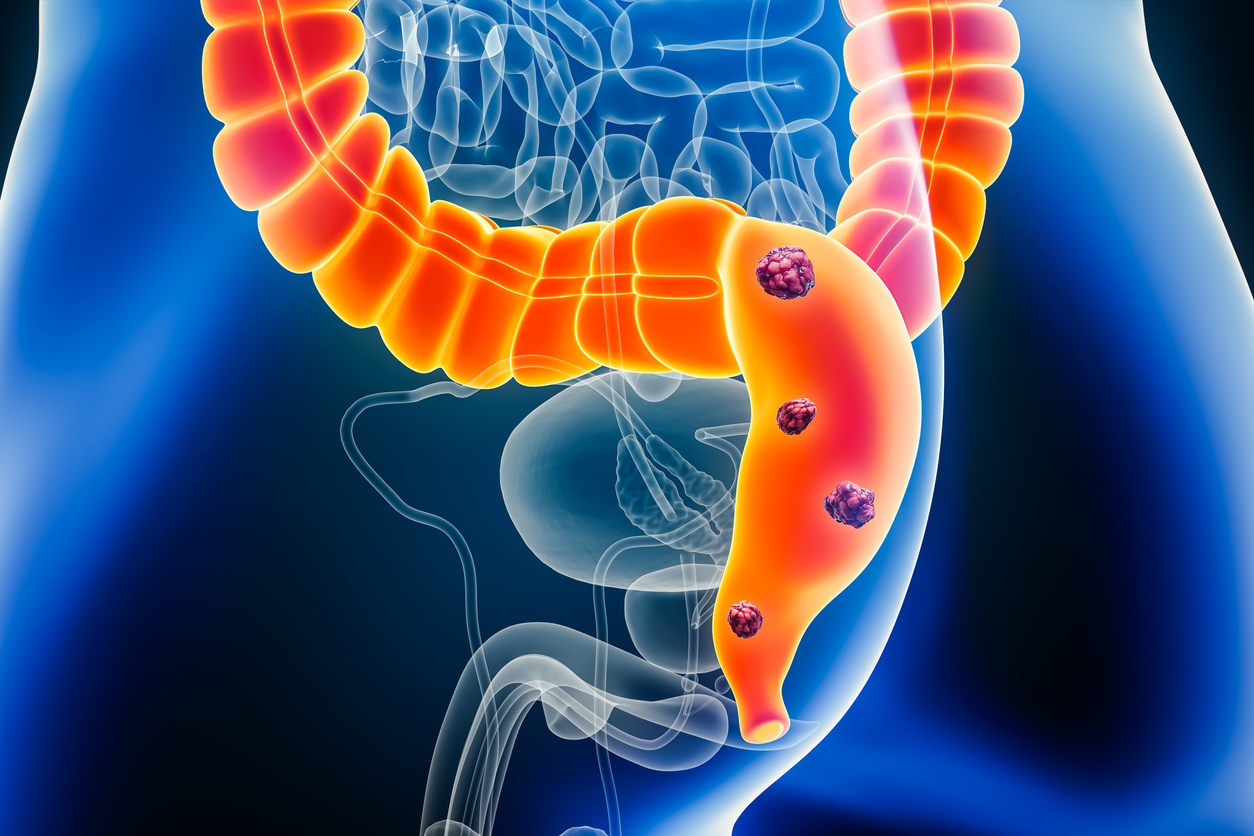
In the randomized Phase 2 trial, investigators studied the efficacy of combining the monoclonal antibody sintilimab with the histone deacetylase inhibitor chidamide (HDACi) with or without the monoclonal antibody bevacizumab in patients with chemotherapy-refractory unresectable colorectal cancer with stable/effective microsatellite mismatch repair (MSS/pMMR). A total of 48 patients were randomly assigned to either the doublet arm (sintilimab and chidamide, n = 23) or the triplet arm (sintilimab, chidamide and bevacizumab, n = 25). The primary endpoint, progression-free survival at 18 weeks, was achieved in 43.8% of patients. The triplet group showed significantly improved results compared with the doublet group.
Last press reviews
Endometrial cancer: Is PARP bringing new hope?
By Ana Espino | Published on February 3, 2026 | 3 min read<br>
Hypertension: a new threshold, a new challenge

By Ana Espino | Published on January 30, 2026 | 3 min read<br><br>...
