2024-02-21
Xeligekimab in moderate to severe plaque psoriasis
Dermatology and Venereology
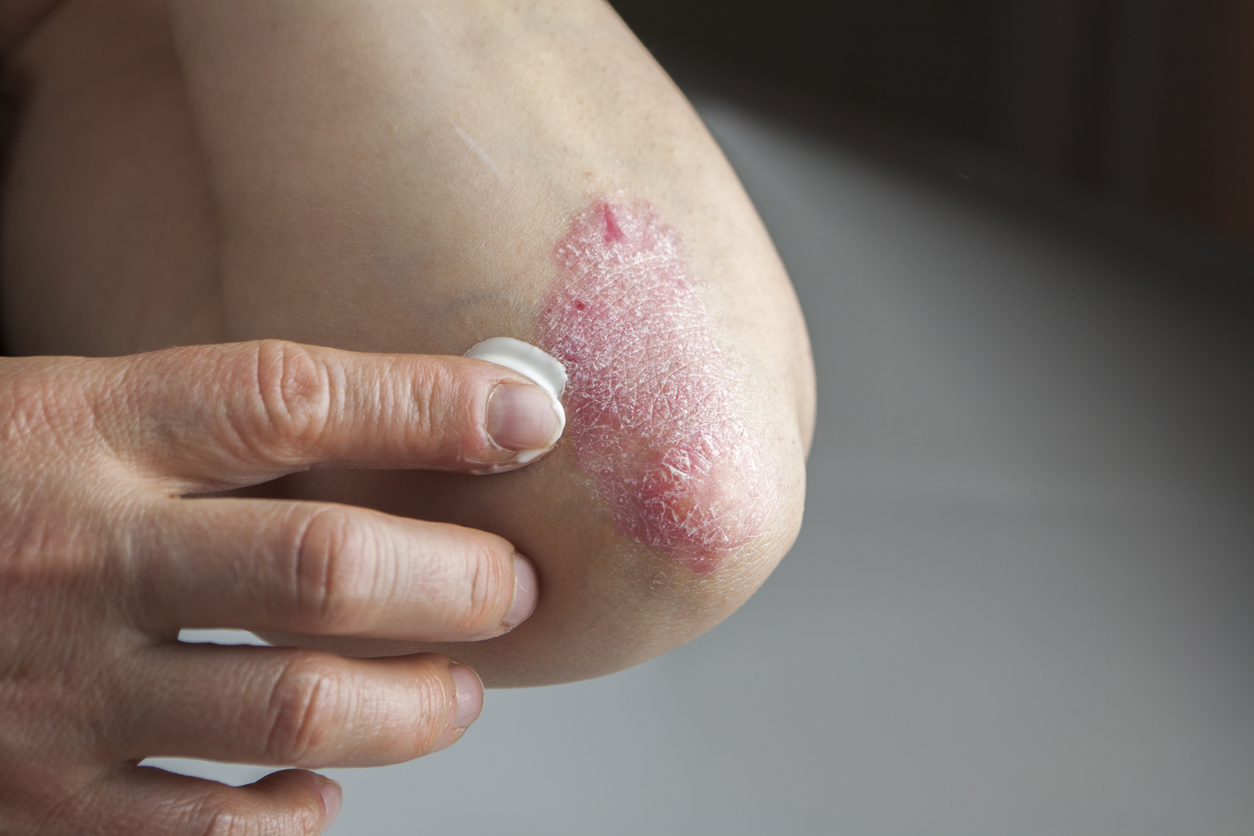
In this study of 420 Chinese patients, researchers evaluated the efficacy and safety of xeligekimab in moderate to severe plaque psoriasis. Xaligekimab is a selective IL-17 inhibitor. Patients were randomized to receive xeligekimab 200 mg every 2 weeks or placebo for an initial period of 12 weeks. Treatment was then continued every 4 weeks for a further 40 weeks. At week 12, PASI scores 75, 90 and 100 were achieved in 90.7%, 74.4% and 30.2% of xeligekimab-treated patients, compared with 8.6%, 1.4% and 0% in the placebo group. No unexpected adverse events were observed.
Last press reviews
RSV: the virus that overwhelms infants
By Ana Espino | Published on March 3rd, 2026 | 3 min read<br><br>
Microbiome and colorectal cancer: the invisible enemy?
By Ana Espino | Published on March 3rd, 2026 | 3 min read<br><br>
Colorectal cancer: a blood test to identify patients at risk of recurrence

By Elodie Vaz | Published on March 2nd, 2026 | 3 min read<br><br>