2024-03-26
Givinostat in Duchenne muscular dystrophy
Neurology
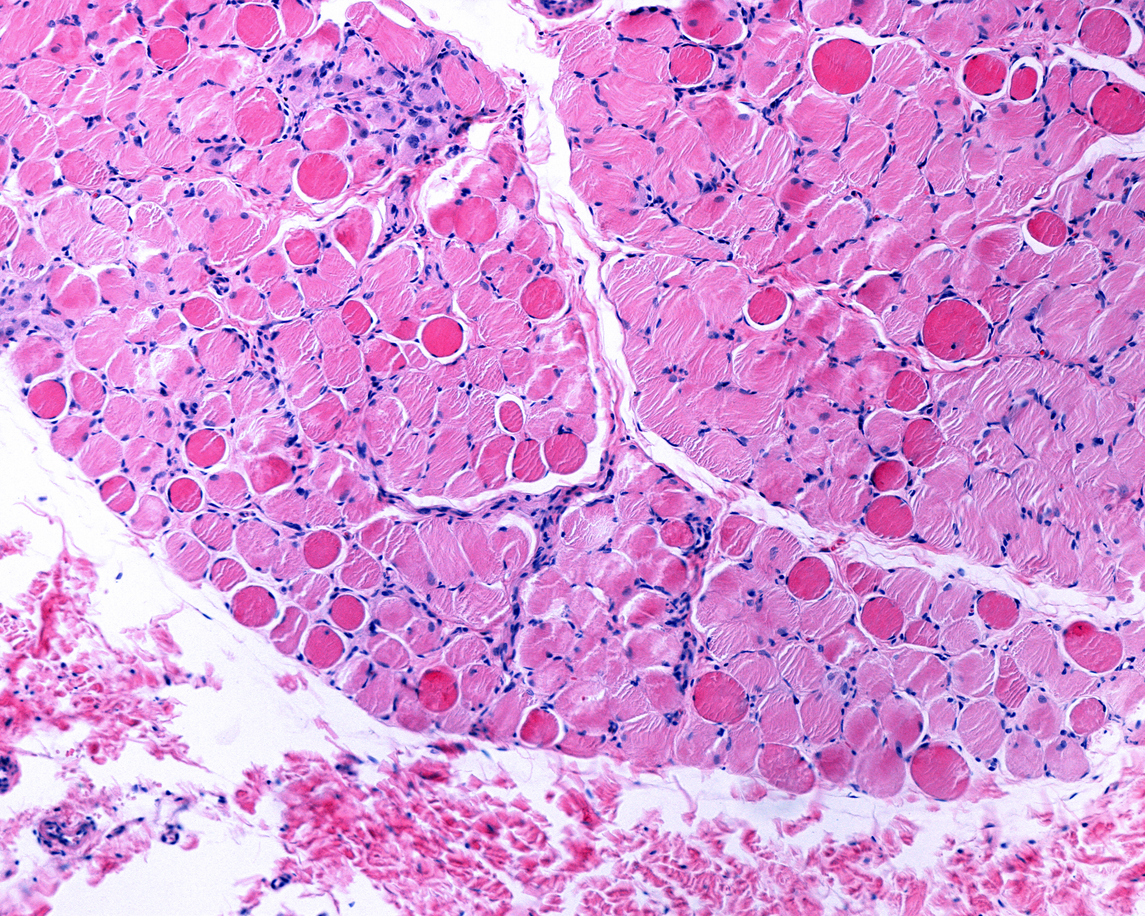
In this Phase 3, multicenter, randomized, double-blind, placebo-controlled clinical trial, researchers evaluated the efficacy and safety of givinostat in the treatment of Duchenne muscular dystrophy. Givinostat is a histone deacetylase inhibitor which may help counter the effects of dystrophin deficiency. The study involved 179 boys with a median age of 9.8 years. Functional abilities, as assessed by the four-step test, deteriorated in both groups of patients. However, the deterioration was less severe with givinostat than with placebo. The most frequent adverse events in the givinostat group were diarrhea and vomiting. An ongoing extension study is evaluating the long-term safety and efficacy of givinostat in patients with Duchenne muscular dystrophy.
Last press reviews
Hypertension: a new threshold, a new challenge

By Ana Espino | Published on January 30, 2026 | 3 min read<br><br>...
Allergies: the molecular revolution is underway

By Ana Espino | Published on January 29, 2026 | 3 min read<br>
